User:Mr. Ibrahem/Methadone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Dolophine, Methadose, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682134 |
| License data | |
| Pregnancy category |
|
| Addiction liability | High[1] |
| Routes of administration | By mouth, intravenous, insufflation, sublingual, rectal |
| Drug class | Opioid |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 15-20% subcutaneous[2] 100% intravenous[2] |
| Protein binding | 85–90%[2] |
| Metabolism | Liver (CYP3A4, CYP2B6 and CYP2D6-mediated)[2][4] |
| Onset of action | Rapid[3] |
| Elimination half-life | 15 to 55 hours[4] |
| Duration of action | Single dose: 4–8 h Prolonged use: • Withdrawal prevention: 1–2 days[3] • Pain relief: 8–12 hours[3][5] |
| Excretion | Urine, faeces[4] |
| Identifiers | |
| |
| Chemical and physical data | |
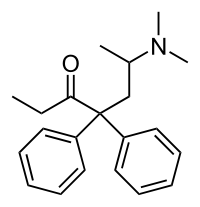
| Formula | C21H27NO |
| Molar mass | 309.453 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Methadone, sold under the brand name Dolophine among others, is an opioid used for opioid maintenance therapy in opioid dependence and for chronic pain management.[3] Detoxification using methadone can be accomplished in less than a month, or it may be done gradually over as long as six months.[3] While a single dose has a rapid effect, maximum effect can take up to five days of use.[3] The pain-relieving effects last about six hours after a single dose.[3][7] After long-term use, in people with normal liver function, effects last 8 to 36 hours.[3][5] Methadone is usually taken by mouth and rarely by injection into a muscle or vein.[3]
Side effects are similar to those of other opioids.[3] These frequently includes dizziness, sleepiness, vomiting, and sweating.[3] Serious risks include opioid abuse and a decreased effort to breathe.[3] Abnormal heart rhythms may also occur due to a prolonged QT interval.[3] The number of deaths in the United States involving methadone poisoning declined from 4,418 in 2011[8] to 3,300 in 2015.[9] Risks are greater with higher doses.[10] Methadone is made by chemical synthesis and acts on opioid receptors.[3]
Methadone was developed in Germany around 1937 to 1939 by Gustav Ehrhart and Max Bockmühl.[11][12] It was approved for use in the United States in 1947.[3] It is on the World Health Organization's List of Essential Medicines.[13] In 2013, about 41,400 kilograms were manufactured globally.[14] It is regulated similarly to other narcotic drugs.[15] It is not particularly expensive in the United States.[16]
References[edit]
- ^ Bonewit-West, Kathy; Hunt, Sue A.; Applegate, Edith (2012). Today's Medical Assistant: Clinical and Administrative Procedures. Elsevier Health Sciences. p. 571. ISBN 9781455701506. Archived from the original on 28 July 2020. Retrieved 2 August 2020.
- ^ a b c d e Anaheim, OM; Moksnes, K; Borchgrevink, PC; Kaasa, S; Dale, O (August 2008). "Clinical pharmacology of methadone for pain". Acta Anaesthesiologica Scandinavica. 52 (7): 879–89. doi:10.1111/j.1399-6576.2008.01597.x. PMID 18331375.
- ^ a b c d e f g h i j k l m n o p q "Methadone Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 23 December 2015. Retrieved 22 December 2015.
- ^ a b c Brown, R; Kraus, C; Fleming, M; Reddy, S (November 2004). "Methadone: applied pharmacology and use as adjunctive treatment in chronic pain" (PDF). Postgraduate Medical Journal. 80 (949): 654–9. doi:10.1136/pgmj.2004.022988. PMC 1743125. PMID 15537850. Archived (PDF) from the original on 2014-05-02.
- ^ a b Toombs, JD; Kral, LA (1 April 2005). "Methadone treatment for pain states". American Family Physician. 71 (7): 1353–8. PMID 15832538. Archived from the original on 5 September 2017.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 28 July 2020. Retrieved 22 September 2020.
- ^ Grissinger, Matthew (August 2011). "Keeping Patients Safe From Methadone Overdoses". Pharmacy and Therapeutics. 36 (8): 462–466. PMC 3171821. PMID 21935293.
- ^ "Data table for Figure 1. Age-adjusted drug-poisoning and opioid-analgesic poisoning death rates: United States, 1999–2011" (PDF). CDC. Archived (PDF) from the original on 23 November 2015. Retrieved 22 December 2015.
- ^ Rudd, Rose A.; Seth, Puja; David, Felicita; Scholl, Lawrence (2016). "Increases in Drug and Opioid-Involved Overdose Deaths — United States, 2010–2015". MMWR. Morbidity and Mortality Weekly Report. 65 (5051): 1445–1452. doi:10.15585/mmwr.mm655051e1. ISSN 0149-2195. PMID 28033313.
- ^ Chou, R; Turner, JA; Devine, EB; Hansen, RN; Sullivan, SD; Blazina, I; Dana, T; Bougatsos, C; Deyo, RA (17 February 2015). "The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop". Annals of Internal Medicine. 162 (4): 276–86. doi:10.7326/M14-2559. PMID 25581257.
- ^ Methadone Matters: Evolving Community Methadone Treatment of Opiate Addiction. CRC Press. 2003. p. 13. ISBN 9780203633090. Archived from the original on 2015-12-23.
- ^ Kleiman, Mark A. R.; Hawdon, James E. (2011). "Diphenypropylamine Derivatives". Encyclopedia of Drug Policy. ISBN 9781506338248. Archived from the original on 2015-12-23.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Narcotic Drugs 2014 (PDF). International Narcotics Control Board. 2015. p. 21. ISBN 9789210481571. Archived (PDF) from the original on 2015-06-02.
- ^ World Health Organization (2009). Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. World Health Organization (WHO). p. 78. hdl:10665/43948. ISBN 9789241547543.
- ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 13. ISBN 9781284057560.
